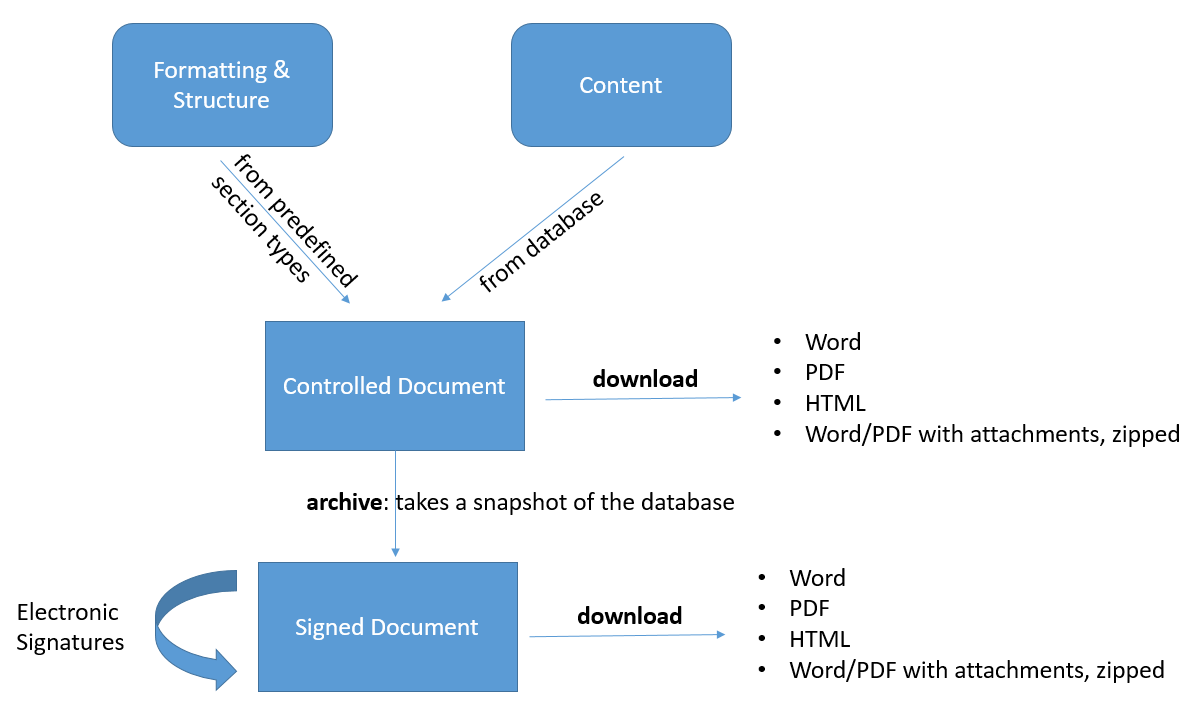
Controlled Documents
Controlled Documents allow you to extract and analyze data from the database in order to archive and electronically sign it as PDF or Word file.
You can
- define what the documents look like by selecting the sections, such as audit trail, purpose, scope, trace tables, list items, etc.
- decide which content goes into the document, e.g. all requirements from one specific folder, or all tests results for a selected set of specifications.
- download and review it as word, pdf or html.
- archive it (this will make sure the content of document will not change even if the items in the database get updated afterwards) by sending them to signature / releasing them.
- electronically sign it (FDA 21 CFR Part 11 compliant).
By default signed documents are created as locked PDFs. This means with PDF editors they cannot be modified without leaving traces. This behavior can be changed in the admin client