QMS Project
General Overview
Data in the QMS project
The default configuration has the following categories.
Note: there can be more or other categories and categories can have other (publishable) fields. Categories can be added and configured in the admin client. Look at the QMS configuration to see how to do it.
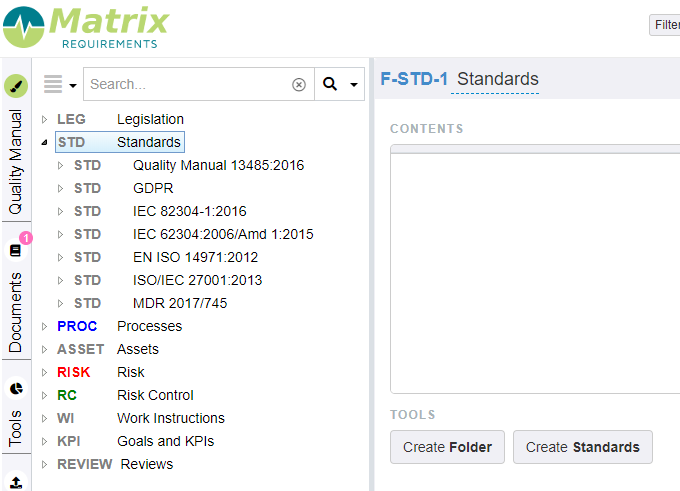
Standards
These items can be found in the folder STD.
This section provides you with the possibility to make a gap analysis of the different standards that are incorporated in your QMS module. E.g. for ISO 13485:2016 you will see the full structure of the standard with a link to the specific processes that relate to specific requirements. You have the possibility to add any other gap analysis.
Processes
These items can be found in the folder PROC.
The processes within the QMS are categorized according to the Standard Operating Procedures (SOPs) they belong to. This allows you to use them as your Quality Manual. The aim is to set up processes specific for your company according to the requirements of the applicable standards such as e.g. ISO 13485:2016. In order to do so, we advise you to consult as well your copy of this standard while creating your own Quality Management System.
The processes have the following structure:
- Plain English: An explanation about the content of this process. This text is to help you define the content of each process, but does not give you the exact content of the standard.
- Review comments/todos: This text box can be used as a tool to communicate between author and reviewer of a specific process.
- Process Input: Different things (legal/regulatory requirements, products, other processes, etc) that serve as input for a process.
- Process Description: description of the process
- Process Output: this section describes the output of the process. This can be specific records, actions, ways of working, etc.
- Responsible for Process Application: Which role(s) have end-responsibilities for the implementation of the process.
- Affected by Process: Which role(s) have to work according to this process or have a direct impact on their work caused by this process.
- Criteria and Methods Needed to Ensure Operation is Effective: This is the effectiveness check. How do you check that this process is effective?
The QMS module comes with already a number of predefined PROC items, based on the requirements of ISO 13485:2016. These items still need to be adapted to your company.
Risks
As it’s required by ISO 13485 and ISO 9001 to do risk analysis on QMS processes as well as on the product, the QMS module has the possibility to document process-related risks.
This risk analysis is based on the principles of Failure Mode End Analysis (FMEA) method. The fields to be completed are:
- Hazard/Hazardous Situation: What could lead to the harm?
- Sequence of events: what are the intermediate steps?
- Harm: What could be the impact of the issue? This is a predefined drop down menu. Depending on which harm you select, automatically the according severity will be selected as well.
- Probability of Occurrence: What is the chance that the Hazard/Hazardous situation occurs?
- Probability of Harm: When the Hazard occurs, what is the chance that the Harm occurs?
- Severity: When the Harm occurs, how bad could the impact be (worst case)?
Based on the Probability of Harm, the Probability of Occurrence and the Severity, the Initial Risk Index is calculated. This is indicated in either green or red. The threshold for green is standard set on 21. The color gives you an indication whether the risk index is fairly low (max. value is 100) or not. It does not give you an indication about whether or not you should add additional risk control measures.
In order to reduce the Risk Index, Risk Control Measures need to be defined. This can be done by either creating a new Risk Control Measure or selecting an existing one. You have to indicate whether the probability or the severity is reduced by the Risk Control Measure and by how much. Based on this the Residual Risk Index will be calculated.
When all Risk Control Measures have been defined and the final Risk Index is calculated, you can indicate that you have reduced the risk as far as possible and that the benefits outweigh the remaining risk.
In addition a justification/conclusion can be added.
Please note that all risk calculations and color indications are customizable.
Risk Controls
When a Risk Control Measure is established, it will be created as a Risk Control Item. This item can be linked to another process and/or the same process as the one to which the risk is linked.
Work Instructions
Work Instructions are supposed to be detailed, company-specific instructions on how to do certain things. They consist of a description field and have the possibility to attach documents.
Publications
This section contains the different publications of the QMS. Whenever all PROCs of a certain SOP with their linked WI are reviewed and approved, they can be published on a webpage (LiveQMS). This webpage can be made accessible to anyone you want within or outside your organization (still password controlled) without giving them direct access to the QMS editor.
Reviews
Reviews allow you to record reviews of - for example - processes or work instruction before publishing them.
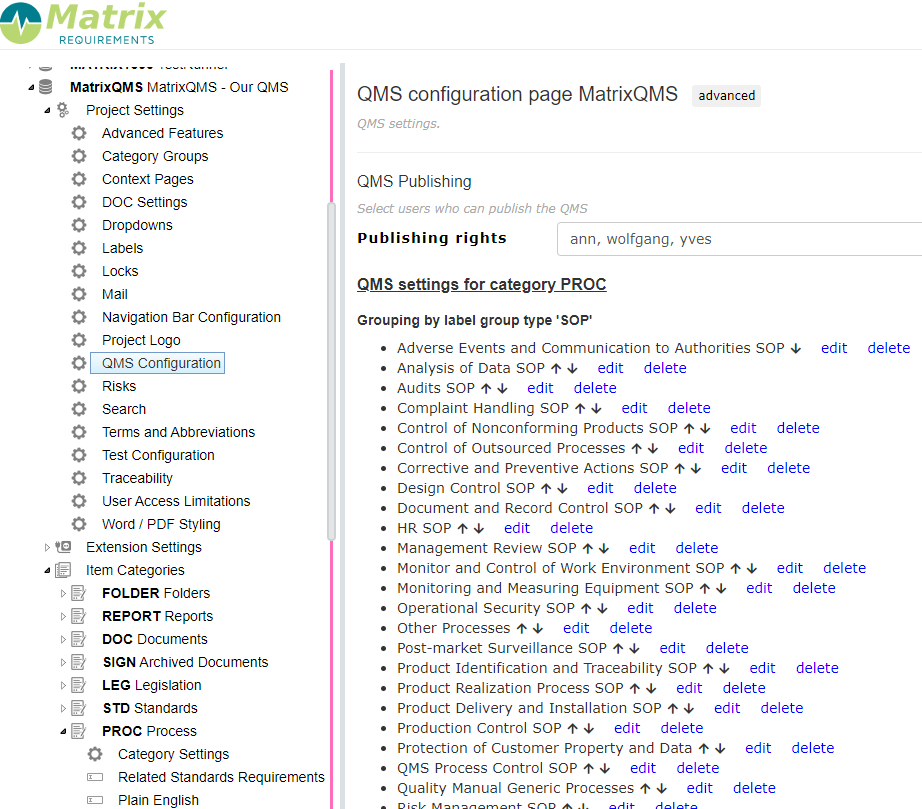
Configuration
In the admin client is a specific setting which allows you to define
- which layers (e.g. procedures and work instructions) you want to publish (in the advanced settings)
- who can publish your procedures and work instructions,
- which labels you can use to to group your procedures and work instructions
- define who can be approve your procedures and work instructions.
Besides that you can per field of a published category decide if it can be published or not. The following field types can be published:
- text fields,
- tables,
- date fields,
- users and
- cross project links.